Life in an aerobic environment constantly results in the formation of reactive oxygen species. Although efficient antioxidant systems have evolved, cells can encounter conditions which exceeds the antioxidant capacity and causes uncontrolled oxidation of proteins, lipids, and nucleic acids (Ulrich and Jakob, 2019). Oxidative protein unfolding is one major threat of oxidative stress and can result in protein aggregation, which is known to be associated with premature aging and neurodegenerative diseases. Therefore, our lab studies the role of stress-sensing chaperones which act as a first line of defense against oxidative unfolding stresses in various organisms (Ulrich et al, 2020).
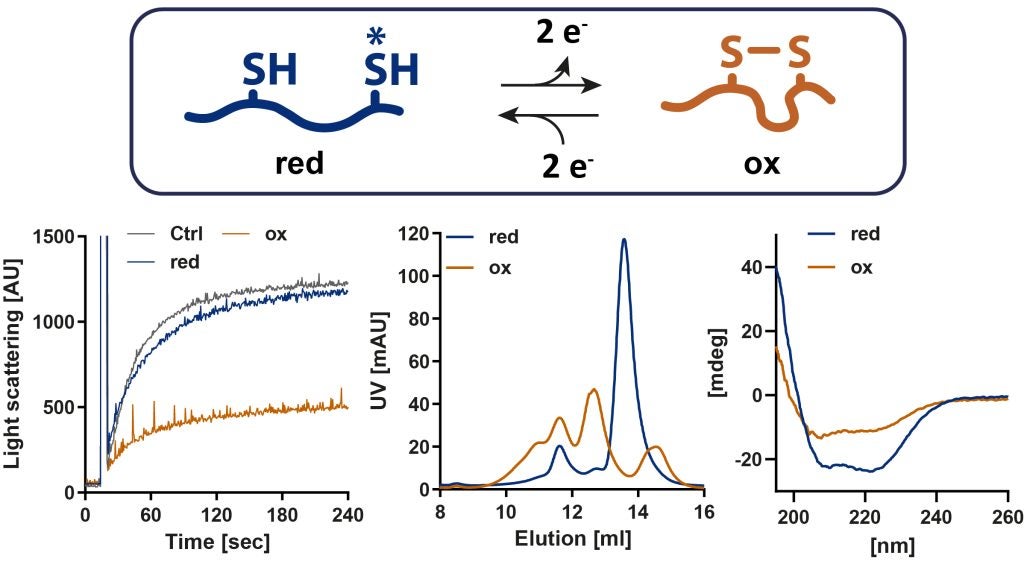
Our lab has discovered the E. coli heat shock protein Hsp33 as first redox-regulated chaperone which becomes activated upon oxidative unfolding stresses in order to bind unfolding protein intermediates and prevent their aggregation (Jakob et al., 1999). Hsp33 is the central player of a redox-regulated chaperone network that efficiently protects cells against the otherwise lethal consequences of oxidative stress. Recently, we identified the cytosolic ATPase Get3 as the Eukaryotic counterpart of Hsp33. Get3 is a dual function protein which under normal conditions targets tail-anchored membrane proteins to the endoplasmic reticulum and turns into an ATP-independent chaperone upon oxidation (Voth et al., 2014). The activation mechanisms depends on the highly orchestrated interplay between its nucleotide-binding state and the redox status of a highly conserved cysteine pair (Ulrich et al. under revision).
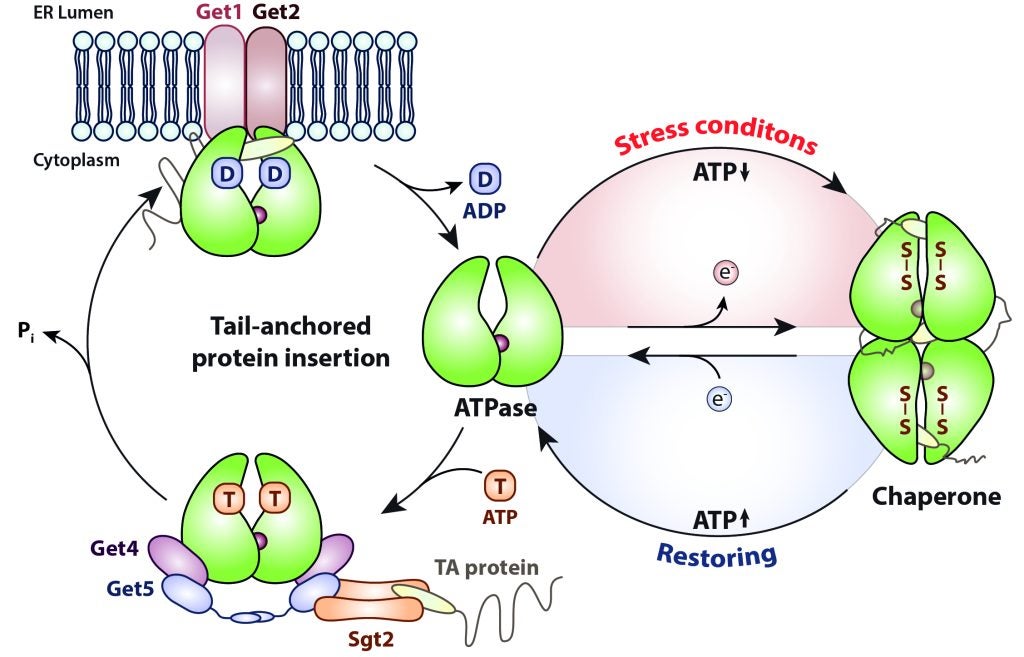
To further understand the physiological role of the stress-dependent switching mechanism, we are studying the redox-regulated chaperone function of its mammalian homolog in cell culture systems exposed to various stress conditions. Structural and functional analysis of the recombinant protein will show whether TRC40 has – in addition to its function as ATPase – indeed redox-regulated chaperone activity.
Contact
Biological Sciences Building
Room 5022
1105 N. University
Ann Arbor, MI 48109
USA
ph | 734.615.1286
lab ph | 734.647.6683
fax | 734.647.0884
News
- U-M researchers discover stress in early life extends lifespan
- Versatile protein helps cells survive oxidative stress
- ICT News Desk: How Bleach Kills Bacteria
- Order out of Disorder: Working Cycle of an Intrinsically Unfolded Chaperone Cell
- The Secret Life of Bleach