A Photosynthetic Organism’s Toolbox for Adapting to Environmental Conditions
The conversion of sunlight into chemical energy by chlorophyll and bacteriochlorophyll and transformation of CO2 into biomass by photosynthetic organisms is an attractive means for production of alternative energy. However, unpredictable sunlight, shading by other chlorophyll-dependent organisms, and the limited 400-700 nm spectral range used by most organisms are limitations to practical use of plant and bacterial photosynthesis for energy generation1,2.
Conveniently, Nature has engineered solutions to each of these shortcomings. Many photosynthetic organisms have developed strategies to persist in environments with intermittent light. One strategy of particular interest to the Bridwell-Rabb Lab is the use of modification enzymes to alter chlorophyll to absorb different types of light. As many of the known chlorophyll and bacteriochlorophyll modification enzymes contain metal ions or metallocofactors such as heme, iron-sulfur clusters, and vitamin B12 (Figure 1), we are interested in understanding the relationship between structure and function in metalloenzyme superfamilies and how the catalytic power of transition metals is exploited to derivatize the chlorophyll/bacteriochlorophyll scaffold.

Figure 1: Chlorophyll and bacteriochlorophyll modification enzymes use metal ions and/or metallocofactors to catalyze impressive chemical transformations. (a) Rieske non-heme iron enzymes use a protein-bound [2Fe-2S] cluster and non-heme iron site to catalyze oxygenation reactions (cofactors from PDB: 3GL2). (b) Vitamin B12 or cobalamin-dependent S-adenosylmethionine radical enzymes use S-adenosylmethionine, a protein-bound [4Fe-4S] cluster, and methylcobalamin to catalyze methylation (cofactors from PDB: 5UL4). In panels a and b protein residues involved in coordinating the cofactors are shown as sticks.
Aside from the exciting chemistry involved in the addition of chemical functionalities to chlorophyll and bacteriochlorophyll, these derivative pigments (Figure 2) each have shifted peak absorption1-3. We are interested in derivatives that extend the absorbance properties of chlorophyll outside of the 400-700 nm spectral range and thus would be advantageous for plants growing under the canopy. Likewise these derivative pigments would be useful in bioreactors because similar to what is observed in algal mats and blooms, moving down in a bioreactor, red light, not absorbed by other chlorophyll-dependent organisms becomes the only light available4,5. Understanding the mechanism and regulation of how organisms tailor their pigments in a habitat-dependent manner presents opportunities to engineer photosynthetic pigments and photosynthetic organisms to more effectively utilize available environmental light.
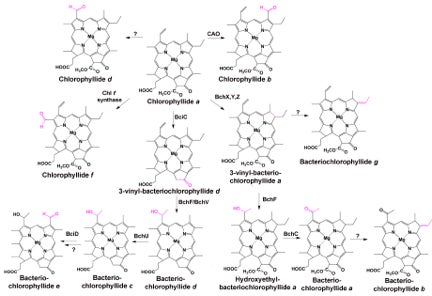
Figure 2: Several derivatives of chlorophyll and bacteriochlorophyll are found in Nature. Each derivative pigment shown here differs from the precursor chlorophyllide a by the enzyme-mediated addition of a chemical functionality on the scaffold (modifications are shown in pink). When known, the enzyme involved in modification is noted above the arrow. Modifications permit fine-tuning of the photosynthetic pigment’s absorbance properties.
References
1. Chen, M. Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annu Rev Biochem 83, 317-340, doi:10.1146/annurev-biochem-072711-162943 (2014).
2. Chen, M. & Blankenship, R. E. Expanding the solar spectrum used by photosynthesis. Trends Plant Sci 16, 427-431, doi:10.1016/j.tplants.2011.03.011 (2011).
3. Chew, A. G. & Bryant, D. A. Chlorophyll biosynthesis in bacteria: the origins of structural and functional diversity. Annu Rev Microbiol 61, 113-129, doi:10.1146/annurev.micro.61.080706.093242 (2007).
4. Kuhl, M. & Jorgensen, B. B. The Light-Field of Microbenthic Communities – Radiance Distribution and Microscale Optics of Sandy Coastal Sediments. Limnol Oceanogr 39, 1368-1398 (1994).
5. Pandey, R., Sahu, A., K, K. V. & M, P. Studies on light intensity distribution inside an open pond photo-bioreactor. Bioprocess Biosyst Eng 38, 1547-1557, doi:10.1007/s00449-015-1398-3 (2015).


