Top panel: Carbonic anhydrase active site. Bottom panel: Scheme of FRET interaction.
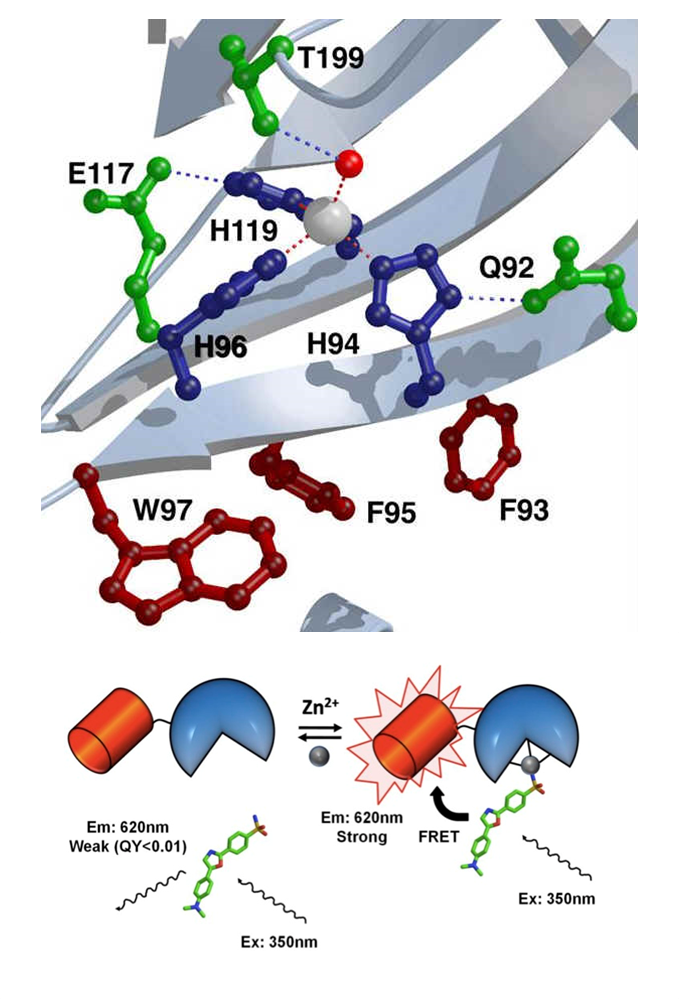
Carbonic anhydrase II (CA) can be used as the transducer in a biosensor to measure zinc, using either exogenous fluorescent sulfonamides or covalently attached fluorophores. CA-based sensors have been developed, covering a wide range of zinc affinities (6 orders of magnitude), kinetics, and specificities. The use of CA to measure free metal ion concentrations in vivo has demanded further improvements to this sensor. These include ratiometric sensors that can either be expressed in the cells or introduced into the cell by a cell importation tag.
The ratiometric sensors use sulfonamides that bind to the holo-enzyme. Upon binding, FRET can occur between the sulfonamides and the DsRed protein fused to the C-terminus of CA. In the Fierke Lab, we are working on altering the metal specificity by mutating the second shell metal ligands, and enhancing the protein stability by introducing a disulfide bond to further optimize CA-based biosensors for the measurement of zinc and copper ions in both in vitro and in vivo conditions.
Collaborators on this project:
Richard Thompson

