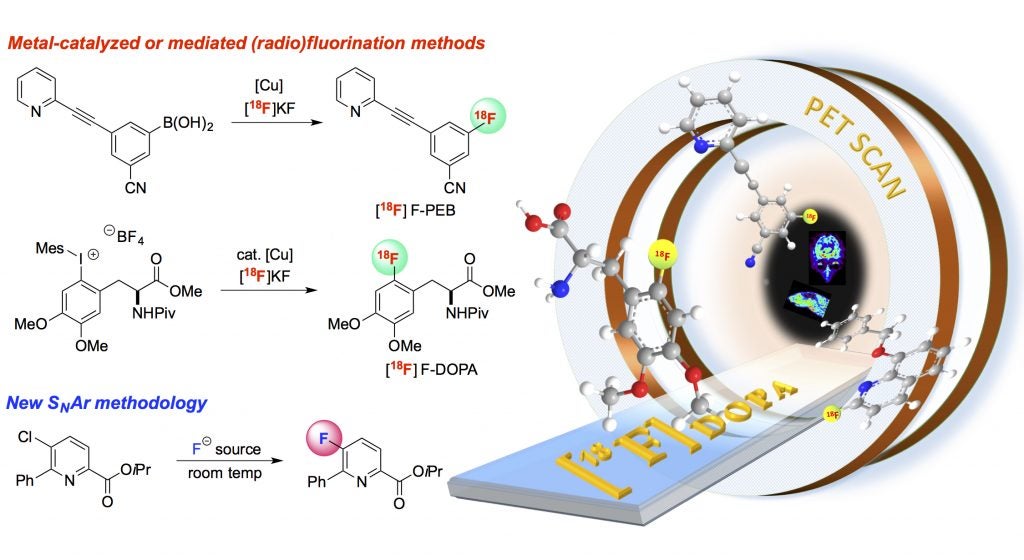
Fluorine-containing molecules are important in the agrochemical, pharmaceutical, and medical imaging sectors, as carbon-fluorine bonds are present in >25% of agrochemicals and pharmaceuticals and >90% of positron emission tomography imaging agents. Despite the prevalence of fluorinated organic molecules, there are still very limited synthetic methods for the selective formation of carbon-fluorine bonds. Challenges in this area include: (1) the requirement for forcing conditions due to the low solubility of alkali metal fluorides in organic solvents; (2) the dearth of metal catalysts for selective C–F coupling reactions; (3) the limited substrate scope of existing C–F coupling reactions; and (4) the slow rate of most fluorination methods, which is problematic for time-sensitive applications like radiofluorination. Our research in this area is focused on addressing these challenges through the development of novel selective, mild, and inexpensive fluorination processes. We also pursue applications of these new methods to the radiofluorination of biologically relevant molecules in collaboration with Prof. Peter Scott (UM Department of Radiology) and to the fluorination of agrochemicals, in collaboration with Dr. Douglas Bland (Dow Chemical Company).
- The development of mild nucleophilic fluorination reactions of aromatic compounds
- The development of metal catalysts for arene and alkane fluorination
- The application of fluorination reactions to radiofluorination
- The development of inexpensive and practical nucleophilic fluoride sources for process-scale applications
Key references:
- Makaravage, K. J.; Brooks, A. F.; Mossine, A. V.; Sanford, M. S.; Scott, P. J. H. “Copper-Mediated Radiofluorination of Arylstannanes with [18F]KF,” Org. Lett. 2016, ASAP.
- Schimler, S. D.; Ryan, S. J.; Bland, D. C.; Anderson, J. E.; Sanford, M. S. “Anhydrous Tetramethylammonium Fluoride for Room-Temperature SNAr Fluorination,” J. Org. Chem. 2015, 80, 12137-12145.
- Mossine, A. V.; Brooks, A. F.; Makaravage, K. J.; Miller, J. M.; Ichiishi, N.; Sanford, M. S.; Scott, P. J. H. “Synthesis of [18F]Arenes via the Copper-Mediated [18F]Fluorination of Boronic Acids,” Org. Lett. 2015, 17, 5780-5783.
- Allen, L. J.; Lee, S. H.; Cheng, Y.; Hanley, P. S.; Muhuhi, J. M.; Kane, E.; Powers, S. L.; Anderson, J. E.; Bell, B. M.; Roth, G. A.; Sanford, M. S.; Bland, D. C. ” Developing Efficient Nucleophilic Fluorination Methods and Application to Substituted Picolinate Esters,” Org. Process Res. Dev. 2014, 18, 1045-1054.
