Currently, MS based assays play a vital role within the drug development pipeline as both a method of screening potential ligands for protein binding and assessing relative or, in some cases, absolute binding strength of a protein-ligand complex. However, obtaining final conformation of ligand binding and structural characterization data (at any level) on the newly-formed complex still remains a significant challenge. This situation frames a critical technology gap in the development phase of pharmaceuticals, where many potential drug candidates are screened by time-consuming or inefficient analytical methods leading to long lead compound development times and, eventually, cost inefficient pharmaceutical products. We are developing IM-MS based protein ligand screening techniques that will enable the acquisition of both binding and structural information simultaneously. In addition, collision induced unfolding (CIU) can provide orthogonal information relative to MS analysis of protein-ligand complexes alone, as the unfolding transitions are very sensitive to the local binding interactions developed between ligand and protein.
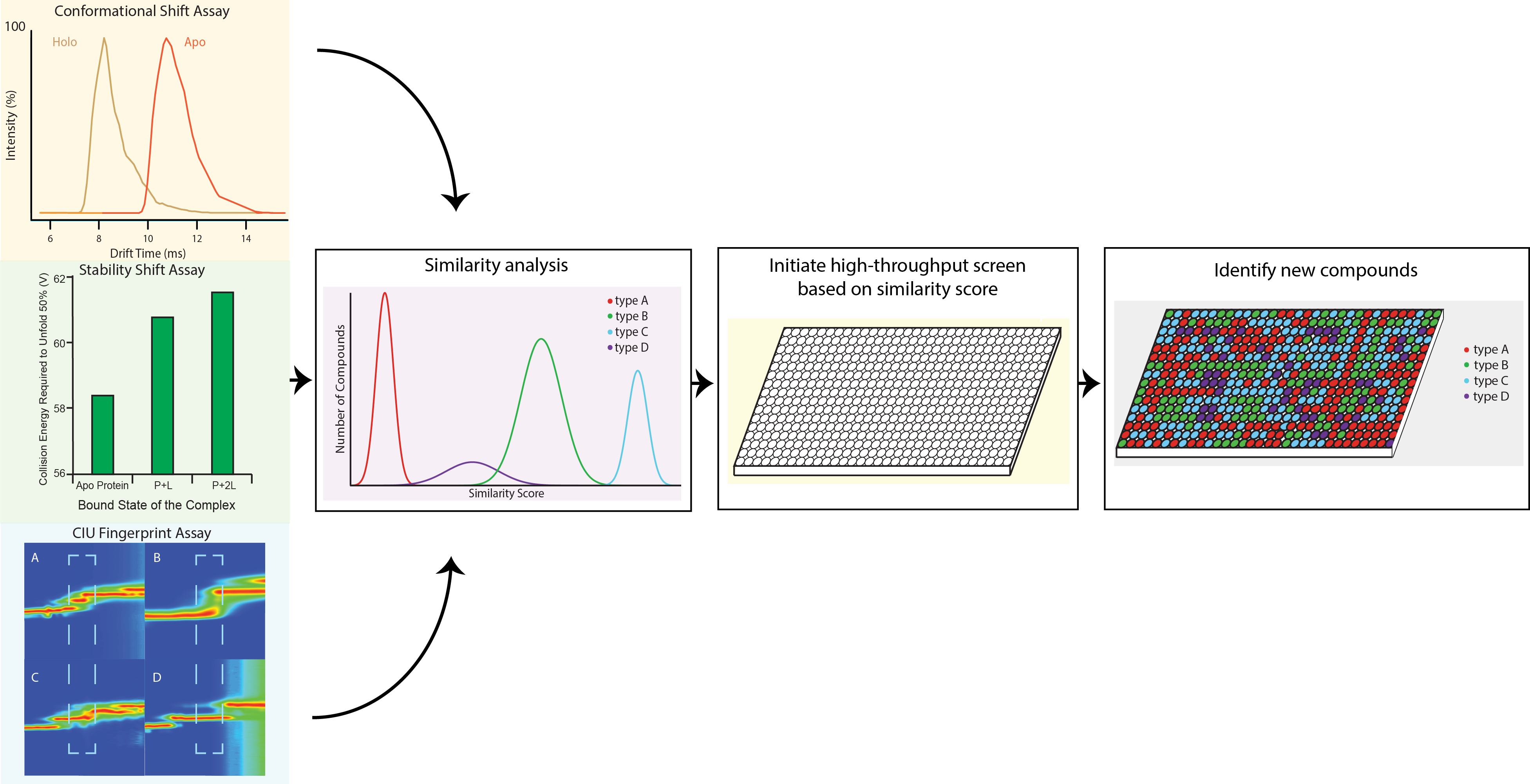
For more details, please refer to Niu, S. et al., Curr. Opinion. Chem. Bio., 2013, 17 (5).
For a detailed summary of current work in this research area, please visit the publication site.
- Welcome Addison, Hanyu, and Ryan!
- Congratulations to Iliana!
- Congratulations to Dr. Rojas Ramirez and Dr. Parson!
- Congratulations to Dr. Han!
- New Publication from the Ruotolo Lab Featured in UM News!
Contact
Ruotolo Group
University of Michigan
Department of Chemistry
Room 4550
930 N. University Ave, Ann Arbor, MI 48109-1055
Phone: (+1) 734-763-2443
Fax: (1+) 734-615-3718
